Oligonucleotides Competitive landscape 2025
DelveInsight’s, “Oligonucleotides Competitive landscape, 2025,” report provides comprehensive insights about 280+ companies and 320+ drugs in Oligonucleotides Competitive landscape. It covers the Oligonucleotides therapeutics assessment by product type, stage, Oligonucleotides route of administration, and Oligonucleotides molecule type. It further highlights the inactive pipeline products in this space.
Geography Covered
- Global coverage
Oligonucleotides Understanding
Oligonucleotides are oligomers composed of repeating nucleotide monomers, comprising deoxyribose or ribose sugar, nitrogenous bases, and a phosphate backbone. Oligonucleotides possess a unique capability to bind specifically to their complements, such as DNA or RNA, leading to the formation of duplexes or, less frequently, higher-order hybrids. This characteristic enables the use of Oligonucleotides as probes for identifying specific DNA or RNA sequences. Oligonucleotide (ON) is a short strand of nucleic acid polymers mostly comprising of thirteen to twenty-five nucleotides, which can hybridize to targeted DNA or RNA. They are categorized into classes including antisense Oligonucleotides (ASOs), small interfering RNA (siRNA), microRNA (miRNAs), and aptamer, which are currently being explored for their use in neurodegenerative disorders, cancer, and even orphan diseases.
They are also undergoing clinical trials for the treatment of dermatological, gastrointestinal, and hormonal disorders. Oligonucleotides can be used to modulate gene expression via a range of processes including RNAi, target degradation by RNase H-mediated cleavage, splicing modulation, non-coding RNA inhibition, gene activation and programmed gene editing. Oligonucleotide therapeutics, including antisense oligonucleotides (ASOs), small interfering RNAs (siRNAs), and splice-switching oligonucleotides, represent a transformative class of drugs with the potential to treat a wide spectrum of genetic and acquired diseases. Despite their promise, the clinical success of these molecules is tightly linked to the development of efficient delivery systems that can overcome their inherent biological limitations. Due to their large molecular weight, hydrophilicity, and negative charge, oligonucleotides face significant barriers in reaching intracellular targets. Additionally, their susceptibility to degradation by nucleases in biological fluids, limited ability to penetrate tissues, rapid renal clearance, and the need for tissue-specific targeting further complicate systemic delivery.
Oligonucleotides have revolutionized diagnostics and therapeutics due to their high specificity, programmability, and ease of synthesis. In diagnostics, they serve as primers in PCR/qPCR, probes in hybridization assays like FISH, and components in advanced platforms like microarrays and CRISPR-based tests. Aptamers enable targeted imaging by binding specific biomarkers, while chemical modifications enhance their stability in vivo. Therapeutically, oligonucleotides include aptamers (e.g., pegaptanib), antisense oligonucleotides (e.g., nusinersen), and siRNAs (e.g., patisiran), targeting diseases by modulating or silencing gene expression. They also underpin mRNA/DNA vaccines, miRNA inhibitors, and CRISPR-based gene editing. Despite challenges like off-target effects and rapid clearance, innovations such as GalNAc conjugation and AI-guided design are improving delivery, specificity, and clinical outcomes, driving progress in precision medicine.
Oligonucleotides Competitive Landscape Report Highlights
- In March 2025, Korro Bio, Inc. a clinical-stage biopharmaceutical company focused on developing a new class of genetic medicines based on editing RNA for both rare and highly prevalent diseases, announced that the US Food and Drug Administration (FDA) has granted orphan drug designation to the investigational medicine KRRO-110 for the treatment of Alpha-1 Antitrypsin Deficiency (AATD).
- In January 2025, Arrowhead Pharmaceuticals, Inc. announced that the US Food and Drug Administration (FDA) had accepted the New Drug Application (NDA) for investigational plozasiran for the treatment of familial chylomicronemia syndrome (FCS), a severe and rare genetic disease.
- In February 2025, AusperBio Therapeutics, Inc. and Ausper Biopharma Co., Ltd. (together AusperBio), a clinical-stage biotechnology company, announced recent progress in the ongoing clinical development of its lead candidate AHB-137, an antisense oligonucleotide (ASO) therapeutic for functional cure of chronic Hepatitis B (CHB).
- In December 2024, Vir Biotechnology, Inc. announced that tobevibart and elebsiran have received U.S. Food and Drug Administration (FDA) Breakthrough Therapy designation and European Medicines Agency (EMA) Priority Medicines (PRIME) designation for the treatment of chronic hepatitis delta (CHD).
- In December 2024, The FDA had granted breakthrough therapy designation to Stoke Therapeutics investigational antisense agent STK-001 for the treatment of genetically confirmed Dravet syndrome (DS), a rare epilepsy disorder.
- In November 2024, Ionis Pharmaceuticals that the US Food and Drug Administration (FDA) had accepted for review the New Drug Application (NDA) for donidalorsen, an investigational RNA-targeted medicine for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adult and pediatric patients 12 years of age and older. The FDA has set an action date of August 21, 2025 under the Prescription Drug User Fee Act (PDUFA).
- In October 2024, Ribocure Pharmaceuticals AB and Suzhou Ribo Life Science Ltd (Ribo) received authorization from the Swedish Medicinal Product Agency (MPA), to initiate a Phase II clinical trial in Sweden with the lipid-lowering siRNA drug RBD5044 that targets APOC3. The trial will evaluate efficacy and safety in patients with mixed dyslipidemia
- In September 2024, WVE-N531, an exon-skipping oligonucleotide developed by Wave Life Sciences for the treatment of Duchenne muscular dystrophy (DMD) in patients amenable to exon 53 skipping, received Orphan Drug Designation from the US Food and Drug Administration (FDA).
- In September 2024, NS Pharma, Inc. (NS Pharma), a subsidiary of Nippon Shinyaku Co., Ltd. (Nippon Shinyaku), announced that the Food and Drug Administration (FDA) has granted rare pediatric disease designation to NS050/NCNP-03 which is being developed for the treatment of Duchenne muscular dystrophy (Duchenne).
- In May 2024, Sunhawk Vision Biotech announced that it has received authorization from the US FDA to commence a Phase II clinical trial for myopia control in children.
- In May 2024, Imvax, Inc., announced the completion of enrollment in its randomized, multicenter, double-blind, placebo-controlled Phase IIb clinical trial of IGV-001 in patients with newly diagnosed glioblastoma (ndGBM).
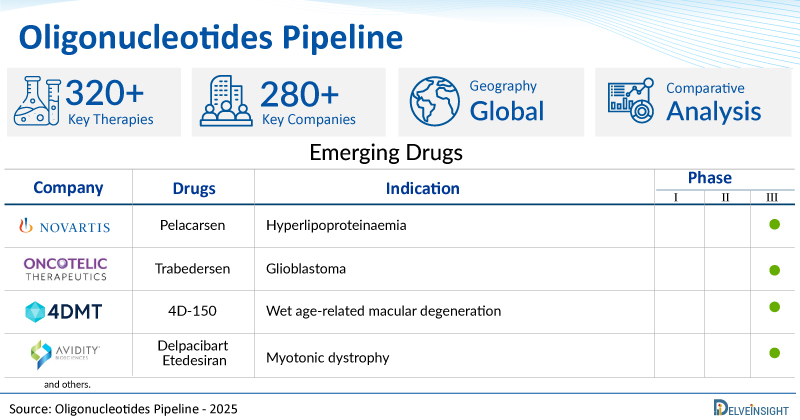
| Oligonucleotides Drugs | Oligonucleotides Companies | Phase | Indication |
| Pelacarsen | Novartis Pharmaceuticals | III | Hyperlipoproteinaemia |
| Trabedersen | Oncotelic | III | Glioblastoma |
| 4D-150 | 4D Molecular Therapeutics | III | Wet age-related macular degeneration |
| Delpacibart Etedesiran | Avidity Biosciences | III | Myotonic dystrophy |
| GSK3228836 | GSK | III | Chronic hepatitis B virus infection |
| WVE-N531 | Wave Life Sciences | II | Duchenne muscular dystrophy |
| ATX-01 | ARTHEx Biotech | II | Myotonic dystrophy Type I & II |
| TAC001 | Tallac Therapeutics | I/II | Solid tumors |
| ATB 301 | Autotelic Bio | I | Pancreatic cancer |
Oligonucleotides Company and Product Profiles (Marketed Therapies)
1. Company Overview: Novartis
Novartis International AG is a global healthcare company based in Switzerland, and one of the largest pharmaceutical companies in the world. Founded in 1996 through the merger of Ciba-Geigy and Sandoz, Novartis is involved in the research, development, manufacturing, and marketing of a broad range of healthcare products, with a focus on pharmaceuticals, generics, and biosimilar. Novartis has committed to sustainable healthcare solutions, including efforts to reduce environmental impact and improve global access to medicines. It also works on initiatives to provide affordable drugs in low- and middle-income countries.
Product Description: LEQVIO
LEQVIO (inclisiran), a first-in-class small interfering RNA (siRNA)-based therapeutic developed by Novartis, is directed to PCSK9 (proprotein convertase subtilisin kexin type 9) mRNA. LEQVIO works differently from other therapies by preventing the production of the target protein in the liver, increasing hepatic uptake of LDL-C, and clearing it from the bloodstream. In 2023, LEQVIO (inclisiran) was approved by MHLW for familial and non-familial hypercholesterolemia and for patients who are at a high risk of developing cardiovascular events. The dosing regimen includes an initial injection, a second dose at three months, followed by maintenance doses every six months. Clinical studies have demonstrated that LEQVIO, in combination with statins, can reduce LDL-C levels by approximately 50%.
2. Company Overview: Company Overview: Astellas Pharma
Astellas Pharma Inc. is a Japanese multinational pharmaceutical company headquartered in Tokyo, known for its innovative approach to healthcare solutions. Established in 2005 through the merger of Yamanouchi Pharmaceutical Co. and Fujisawa Pharmaceutical Co., Astellas focuses on areas such as oncology, urology, immunology, and neuroscience. The company is committed to improving patient outcomes by advancing therapies that address unmet medical needs, with a significant emphasis on research and development. Astellas also actively pursues global partnerships and collaborations to accelerate drug discovery and expand its market presence across key regions, including North America, Europe, and Asia.
Product Description: IZERVAY
Avacincaptad pegol, marketed under the brand name IZERVAY, is an approved medication specifically designed for the treatment of geographic atrophy. Avacincaptad pegol is an RNA aptamer that is covalently linked to a branched polyethylene glycol (PEG) molecule. Its primary function is to inhibit complement factor C5, a key component in the complement cascade involved in inflammatory processes associated with AMD. By blocking the cleavage of C5 into its active fragments (C5a and C5b), avacincaptad pegol aims to reduce inflammation and slow the progression of GA. IZERVAY was approved by the US Food and Drug Administration on August 4, 2023, for the treatment of GA secondary to AMD and is currently under review by the European Medicines Agency. The drug is also being evaluated for the treatment of Stargardt disease.
3. Company Overview: Company Overview: Alnylam Pharmaceuticals
Alnylam Pharmaceuticals is a biopharmaceutical company headquartered in Cambridge, Massachusetts, focused on the discovery, development, and commercialization of RNA interference (RNAi) therapeutics. Founded in 2002, the company is a recognized pioneer in RNAi technology, which enables the silencing of specific genes associated with disease. Alnylam's pipeline targets a range of rare genetic, cardio-metabolic, and hepatic infectious diseases, with several approved products, including ONPATTRO®, GIVLAARI®, OXLUMO®, and AMVUTTRA®. The company maintains a strong emphasis on scientific innovation, strategic partnerships, and global expansion, aiming to transform the treatment landscape for patients with limited therapeutic options.
Product Description: AMVUTTRA
AMVUTTRA® (vutrisiran) is an RNAi therapeutic that delivers rapid knockdown of transthyretin (TTR), addressing the underlying cause of transthyretin (ATTR) amyloidosis. Administered quarterly via subcutaneous injection by a healthcare professional, AMVUTTRA is approved and marketed in more than 15 countries for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis (hATTR-PN) in adults and is approved in the U.S. for the treatment of the cardiomyopathy of wild-type or hereditary transthyretin-mediated amyloidosis (ATTR-CM) in adults to reduce cardiovascular mortality, cardiovascular hospitalizations and urgent heart failure visits.
4. Company Overview: Company Overview: Ionis Pharmaceuticals
Ionis Pharmaceuticals, Inc., founded in 1989 and headquartered in Carlsbad, California, is a biotechnology company specializing in RNA-targeted therapeutics. The company has been a pioneer in the development of antisense technology, aiming to address a broad range of diseases, including neurological disorders, cardiovascular conditions, and rare diseases. Ionis focuses on discovering and developing RNA-based therapies using its proprietary platform, which includes advanced antisense and RNA interference (RNAi) technologies. The company emphasizes precision medicine and partners with major pharmaceutical firms to advance its pipeline. With a diversified portfolio of wholly owned and partnered programs, Ionis continues to expand its presence in the biopharmaceutical sector. It is publicly traded on the Nasdaq and remains focused on advancing RNA-targeted approaches to deliver transformative therapies to patients.
Product Description: TEGSEDI
Tegsedi (inotersen) is an antisense oligonucleotide therapy designed to treat adults with hereditary transthyretin-mediated amyloidosis (hATTR) experiencing stage 1 or 2 polyneuropathy. It functions by reducing the production of transthyretin (TTR) protein, thereby slowing the progression of nerve damage. Administered as a 284 mg subcutaneous injection once weekly, Tegsedi requires regular monitoring due to potential serious side effects, including low platelet counts and kidney inflammation. Common side effects encompass injection site reactions, nausea, headache, fatigue, and fever. In the United States, Tegsedi is available only through a restricted program called the Tegsedi Risk Evaluation and Mitigation Strategy (REMS) Program to ensure safe use. TEGSEDI is a once weekly, self-administered subcutaneous medicine approved in the U.S., Europe, Canada and Brazil for the treatment of patients with ATTRv-PN.
Oligonucleotides Company and Product Profiles (Pipeline Therapies)
1. Company Overview: Novartis Pharmaceuticals
Novartis Pharmaceuticals is a leading global healthcare company headquartered in Switzerland, dedicated to reimagining medicine to improve and extend people’s lives. Operating through a robust portfolio that includes innovative prescription medicines, Novartis focuses on key therapeutic areas such as oncology, immunology, neuroscience, and cardiovascular disease. The company leverages advanced science and digital technologies to deliver high-value treatments while maintaining a strong commitment to research and development. With a presence in over 150 countries, Novartis continues to drive sustainable growth through strategic partnerships, a deep pipeline of novel therapies, and a clear focus on operational excellence and patient-centric innovation.
Product Description: Pelacarsen
Pelacarsen (TQJ230), also known as IONIS-APO(a)-LRx, AKCEA-APO(a)-LRx, and TQJ230, is an investigational antisense medicine designed to reduce apolipoprotein(a) in the liver to offer a direct approach for reducing lipoprotein(a), or Lp (a) - a very atherogenic and thrombogenic form of LDL. Elevated Lp (a) is recognized as an independent genetic cause of coronary artery disease, heart attack, stroke, and peripheral arterial disease. Pelacarsen, an investigational antisense medicine designed to lower Lp(a), was discovered by Ionis and licensed to Novartis in 2019. Currently the drug is in Phase III for the treatment of Hyperlipoproteinaemia.
2. Company Overview: Company Overview: Oncotelic
Oncotelic Therapeutics is a clinical-stage biopharmaceutical company focused on developing innovative therapies for cancer and rare diseases. Headquartered in Agoura Hills, California, the company leverages RNA therapeutics and immuno-oncology approaches to target difficult-to-treat tumors and fibrotic conditions. Its lead programs include treatments for glioblastoma, melanoma, and idiopathic pulmonary fibrosis, among others. Oncotelic integrates expertise in TGF-ß inhibition and oligonucleotide therapeutics to modulate disease pathways with precision. Through a combination of in-house R&D and collaborative partnerships, the company aims to deliver impactful therapies that address significant unmet medical needs.
Product Description: Trabedersen
OT-101, also referred to Trabedersen, is a novel antisense oligodeoxynucleotide (ODN) developed by Oncotelic for the treatment of patients with pancreatic carcinoma, malignant melanoma, colorectal carcinoma, high-grade glioma (HGG), and other transforming growth factor beta 2 (TGF-ß2) overexpressing malignancies (e.g., prostate carcinoma, renal cell carcinoma, etc.). Trabedersen is a synthetic 18-mer phosphorothioate oligodeoxynucleotide (S-ODN) complementary to the messenger ribonucleic acid (mRNA) of the human TGF-ß2 gene. Cancers overexpress TGF-ß, which suppresses host innate immune response to the cancers. Treatment with OT-101 lifts the TGF-ß cloaking effect and allows innate or therapeutic immunity to attack and eliminate the cancers. The company had completed Phase II for pancreatic cancer and melanoma, and Phase II in glioblastoma with robust efficacy and safety.
3. Company Overview: Company Overview: Wave Life Sciences
Wave Life Sciences is a biotechnology company focused on unlocking the broad potential of RNA medicines to transform human health. Wave’s RNA medicines platform, PRISM®, combines multiple modalities, chemistry innovation and deep insights in human genetics to deliver scientific breakthroughs that treat both rare and prevalent disorders. Its toolkit of RNA-targeting modalities includes editing, splicing, RNA interference and antisense silencing, providing Wave with unmatched capabilities for designing and sustainably delivering candidates that optimally address disease biology. Wave’s diversified pipeline includes clinical programs in Duchenne muscular dystrophy, Alpha-1 antitrypsin deficiency and Huntington’s disease, as well as a preclinical program in obesity. Driven by the calling to “Reimagine Possible”, Wave is leading the charge toward a world in which human potential is no longer hindered by the burden of disease.
Product Description: WVE-N531
WVE-N531 is an exon skipping oligonucleotide being developed as a disease modifying treatment for boys with Duchenne muscular dystrophy amenable to exon 53 skipping. WVE-N531 was designed using Wave’s best-in-class oligonucleotide chemistry modifications, including PN backbone chemistry. WVE-N531 has received Orphan Drug Designation and Rare Pediatric Disease Designation from the U.S. Food & Drug Administration. Currently, the drug is in Phase II stage of Clinical trial evaluation for the treatment of Myotonic dystrophy.
4. Company Overview: Company Overview: Autotelic Bio
Autotelic Bio is a clinical-stage South Korean biotechnology company founded in 2015, specializing in the development of next-generation nucleic acid-based therapeutics, particularly antisense oligonucleotides (ASOs). Utilizing proprietary platforms like ASODE and CATs, the company aims to deliver targeted and optimized ASO therapies with enhanced efficacy and safety profiles. Its diversified pipeline includes candidates for oncology, idiopathic pulmonary fibrosis (IPF), and fixed-dose combinations for metabolic disorders. As of March 2022, Autotelic Bio has secured USD 18.2 million in funding, with a USD 12.6 million Series B round led by investors such as UTC Investment and Stonebridge Ventures. The company aspires to become a global leader in cancer treatment by addressing previously undruggable targets.
Product Description: ATB 301
ATB-301 is an investigational antisense oligonucleotide (ASO) therapy developed by Autotelic Bio, targeting Transforming Growth Factor Beta 2 (TGF-ß2). It is being evaluated in combination with recombinant interleukin-2 (Aldesleukin) for the treatment of advanced or metastatic solid tumors, including pancreatic cancer and renal cell carcinoma. The therapy aims to inhibit TGF-ß2 to modulate the tumor microenvironment and enhance immune responses. The drug demonstrated suppression of tumor growth and increase of CD8 T cells in cancer tissue through inhibition of TGF-ß2 in pancreatic cancer model. Currently, the drug is in Phase I stage of Clinical trial evaluation for the treatment of Pancreatic Cancer.
5. Company Overview: Company Overview: Tallac Therapeutics
Tallac Therapeutics is a clinical-stage biopharmaceutical company based in California, focused on developing novel immunotherapies for cancer. The company’s proprietary Toll-like receptor agonist antibody conjugate (TAAC) platform is designed to stimulate innate and adaptive immune responses directly within the tumor microenvironment. Tallac’s lead programs aim to activate Toll-like receptor 9 (TLR9) in a targeted manner, with the goal of enhancing anti-tumor immunity while minimizing systemic toxicity. By leveraging its platform to create next-generation immuno-oncology treatments, Tallac is advancing a pipeline of therapeutic candidates intended to address various solid tumors and improve patient outcomes.
Product Description: TAC001
TAC-001 is a novel antibody-oligonucleotide conjugate designed to deliver systemic TLR9 agonism with targeted immune activation of B cells, which plays a key role in cancer immunity. In preclinical studies, systemically administered TAC-001 is active as a single agent across a number of syngeneic tumor models including ones with immune suppression and resistance, leading to complete tumor eradication and immune memory.
6. Company Overview: Company Overview: ARTHEx Biotech
ARTHEx Biotech is a clinical-stage biotechnology company focused on developing innovative medicines through the modulation of gene expression. The Company’s lead investigational compound, ATX-01, is being evaluated for the treatment of myotonic dystrophy type 1 (DM1), a rare neuromuscular disorder, in the Phase I-IIa ArthemiR™trial. ArthemiR trial is co-funded by EIC Accelerator program under the Grant Agreement Nº 190181217. ARTHEx is advancing its in-house discovery engine to identify and develop nucleic acid-based therapies for other disorders with high unmet medical need, including genetically-driven diseases. The Company headquarters are in Valencia, Spain.
Product Description: ATX-01
ATX-01 is an antimiR oligonucleotide designed to target microRNA 23b (miR-23b), which is involved in the pathogenesis of DM1. It has been demonstrated, in human DM1 myoblast cell lines and in two murine models, that ATX-01 has a unique, dual mechanism of action which reduces toxic DMPK mRNA and increases MBNL protein production. ATX-01 was discovered through ARTHEx’s in-house discovery engine, which was built to identify, design and optimize novel gene expression modulators and ensure their preferential delivery to target tissues affected by the disease. Currently the drug is in Phase I/II stage of its clinical development for the treatment of Myotonic Dystrophy Type 1.
Further product details are provided in the report……..
Oligonucleotides Analytical Perspective by DelveInsight
In-depth Commercial Assessment: Oligonucleotides Collaboration Analysis by Companies
The Oligonucleotides competitive landscape Report provides in-depth commercial assessment of drugs that have been included, which comprises collaboration, agreement, licensing and acquisition – deals values trends. The sub-segmentation is described in the report which provide company-company collaboration (licensing/partnering), company academic collaboration and acquisition analysis in tabulated form.
Oligonucleotides Competitive Landscape
The Oligonucleotides competitive landscape report comprises of comparative assessment of Companies (by therapy, development stage, and technology).
Oligonucleotides Report Assessment
- Oligonucleotides Company Analysis
- Oligonucleotides Therapeutic Assessment
- Oligonucleotides Pipeline Assessment
- Inactive drugs assessment
- Unmet Needs
Key Questions
Current Treatment Scenario and Emerging Therapies:
- How many companies are developing Oligonucleotides drugs?
- How many Oligonucleotides drugs are developed by each company?
- How many emerging Oligonucleotides drugs are in mid-stage, and late-stage of development for the treatment of Oligonucleotides?
- What are the key collaborations (Industry–Industry, Industry–Academia), Mergers and acquisitions, licensing activities related to the Oligonucleotides therapeutics?
- What are the recent trends, drug types and novel technologies developed to overcome the limitation of existing therapies?
- What are the clinical studies going on for Oligonucleotides and their status?
- What are the key designations that have been granted to the emerging and approved Oligonucleotides drugs?
Get detailed insights @ DelveInsight Blogs