Table of Contents
Peripheral T-cell Lymphoma (PTCL) is a diverse disorder category that accounts for 10% to 15% of non-Hodgkin lymphomas. Most PTCL subtypes, including PTCL-NOS, AITL, ALCL, enteropathy-type T-cell lymphoma, and extranodal natural killer (NK) cell/T-cell lymphoma, are aggressive (fast-growing) lymphomas.
PTCLs are rare in the United States but more prevalent in Asia, Africa, and the Caribbean, probably as a result of infection to certain viruses such as the Epstein-Barr virus (EBV) and the human T-cell leukemia virus-1 (HTLV-1). PTCLs often afflict adults over 60; however, PTCLs are also diagnosed in young adults and children.
As per DelveInsight analysis, the total peripheral T-cell lymphoma incidence in the 7MM was 18K in 2021, growing at a CAGR of 1.1% during the study period (2019–2032). United States accounted for 6.3K peripheral T-cell lymphoma incident cases in 2021.
Delveinsight also estimated that among EU-5, the highest cases of the incident population were found to be in Germany in 2021, followed by France. The least population with PTCL in 2021 was found to be in Spain.
Patients with PTCL benefit from a treatment plan that is tailored to their specific symptoms, diagnosis, and reaction to various medications. When conservative peripheral T-cell lymphoma treatment options are used, many cases of PTCL resolve within a few weeks. This, however, is not the case for all patients. For other people, PTCL can persist considerably longer, even for months.
Current Peripheral T-cell Lymphoma Treatment Space
The peripheral T-cell lymphoma treatment is aimed at curing the disease and includes the use of combination chemotherapy regimens, localized radiotherapy, stem cell transplants, and steroid treatment, among other things. It is usually treated with a combination of chemotherapies such as CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) and CHOEP or EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone). In certain circumstances, a stem cell or bone marrow transplant is suggested after the completion of combined chemotherapy.
The peripheral T-cell lymphoma treatment market currently offers supporting treatment choices such as conservative (nonsurgical) and surgical approaches. Physical therapy, behavioral treatments, and pharmaceutical therapies are also used in conservative peripheral T-cell lymphoma treatment.
According to the analysis, 80-90% of patients are cured with current peripheral T-cell lymphoma therapies (pain relievers, muscle relaxants, anti-inflammatories, antidepressants, calcium channel 2-ligands, epidural steroids, opioids, topical pain medications, physical therapy, and behavioral therapy) within 6-12 weeks or one year of PTCL treatment, which are considered the first line of treatment for PTCL patients.
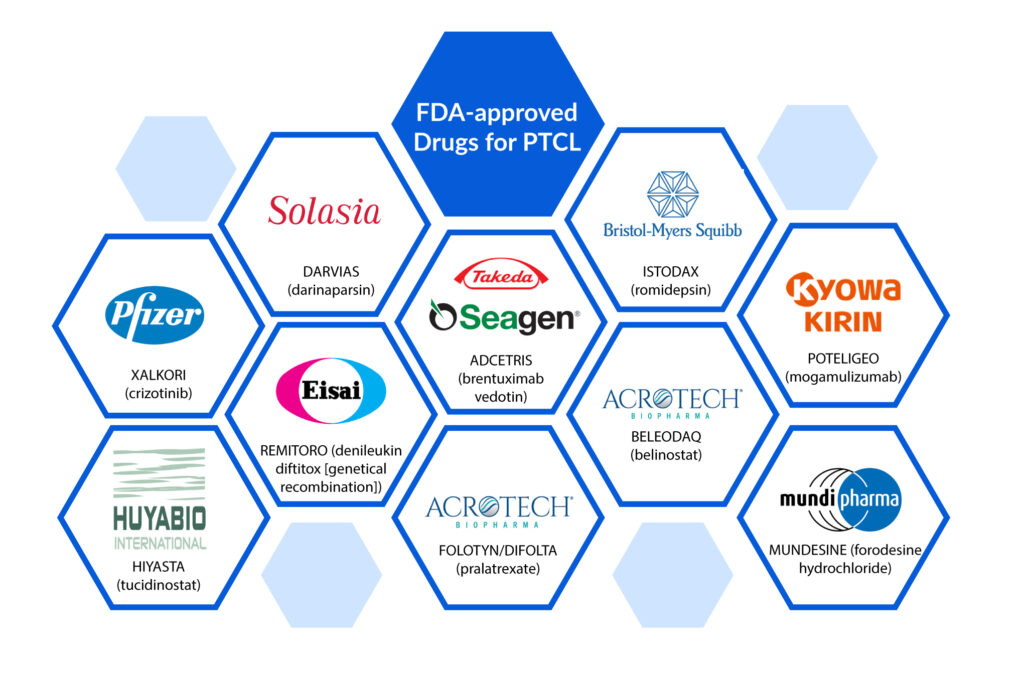
BELEODAQ (Belinostat, Acrotech Biopharma), ISTODAX (Romidepsin, Celgene), FOLOTYN (Pralatrexate, Acrotech Biopharma), ARRANON (Nelarabine, GlaxoSmithKline), and ADCETRIS (Brentuximab vedotin, Seattle Genetics) are among the FDA-approved peripheral T-cell lymphoma treatments.
Apart from the approved PTCL therapies, there are also off-label therapies and additional new therapeutics for peripheral T-cell lymphoma treatment. Off-label therapy for relapsed peripheral T-cell lymphoma treatment that has been listed in the NCCN guidelines includes MabThera (Rituxan), Bortezomib, Bendamustine, Lenalidomide, and Alemtuzumab.
Moreover, recently approved drugs for PTCL treatment include XALKORI (crizotinib) by Pfizer, which was recently approved in 2021 for children patients 1 year and older and young adults with relapsed or refractory, systemic ALK-positive ALCL, and HIYASTA (tucidinostat) by HUYA Bioscience International. Furthermore, the Japanese Pharmaceuticals and Medical Devices Agency approved HBI-8000 monotherapy for relapsed or refractory (R/R) ATL on June 23, 2021.
Additionally, Eisai recently announced in March 2021 that it had obtained manufacturing and marketing approval in Japan for the anticancer agent “Remitoro for intravenous drip infusion 300g” (denileukin diftitox (genetic recombinant)) with the indications of relapsed or refractory (r/r) peripheral T-cell lymphoma and r/r cutaneous T-cell lymphoma. Moreover, on May 19, 2021, REMITORO was added to Japan’s National Health Insurance Drug Price List.
Another drug DARVIAS (darinaparsin), developed by Solasia Pharma, was recently approved by the MHLW for relapsed or refractory PTCL in June 2022. Furthermore, the company reported that DARVIAS Injection was launched in Japan on August 22, 2022, for relapsed or refractory peripheral T-cell lymphoma treatment.
However, one of the most difficult challenges for novel therapies is the capacity to conduct robust clinical studies in rare diseases. Leaving aside these difficulties, recent advances in understanding the biology and pathology of PTCL have resulted in the development of novel therapies.
With the new insights into understanding PTCL, future efforts for PTCL treatment should focus on establishing trials customized for distinct subtypes and investigating innovative combination treatments in the front-line scenario.
Emerging Therapies for Peripheral T-cell Lymphoma Treatment
The peripheral T-cell lymphoma pipeline possesses potential drugs in the mid-stage of development and are expected to be launched in the coming years. Presently, leading companies such as Viracta Therapeutics, Dizal Pharmaceuticals, Daiichi Sankyo, CStone Pharmaceuticals, Rhizen Pharmaceuticals, Kura Oncology, BeiGene, Celgene Corporation, Innate Pharma, Otsuka Pharmaceutical, Astex Pharmaceuticals, Merck Sharp & Dohme Corp., Bristol Myers Squibb, Affimed, AstraZeneca, and others are working in the domain and will create a significant positive shift in PTCL market size in the future.
Additionally, various emerging therapies are being evaluated in clinical trials for peripheral T-cell lymphoma treatment and its subtypes. The emerging peripheral T-cell lymphoma therapies include Nanatinostat + Valganciclovir, COPIKTRA, Tolinapant, Valemetostat, AZD4573, and others.
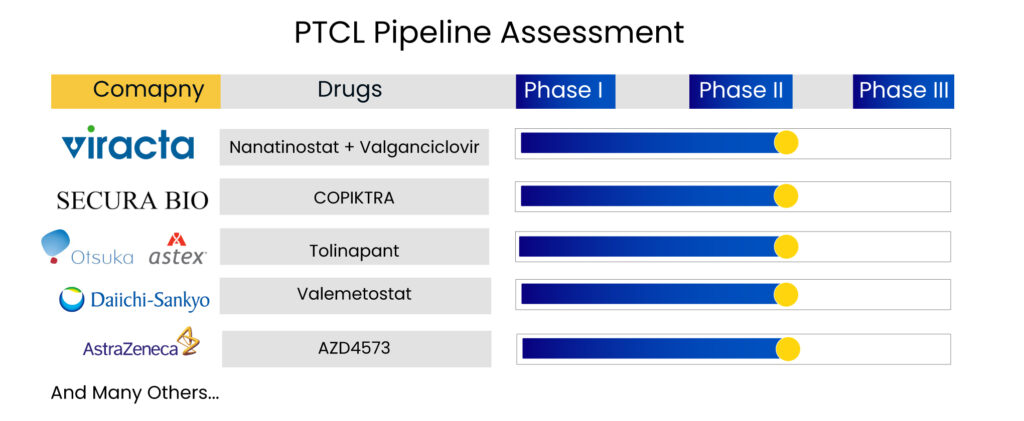
Viracta Therapeutics is developing nanatinostat, an orally accessible histone deacetylase (HDAC) inhibitor. It is selective for certain isoforms of Class I HDACs, which is important for activating viral genes that are epigenetically repressed in Epstein-Barr virus (EBV)-associated cancers. Multiple PTCL clinical trials are now underway to examine the effectiveness of nanatinostat with valganciclovir in patients with relapsed/refractory EBV + malignancies. Currently, nanatinostat is being studied in conjunction with the antiviral drug valganciclovir as an all-oral combination treatment in multiple subtypes of EBV-associated cancers.
Ongoing trials include a pivotal, global, multicenter, open-label Phase II basket study in several subtypes of relapsed/refractory EBV+ lymphoma (NAVAL-1) and a multinational Phase Ib/II trial in patients with EBV+ recurrent or metastatic nasopharyngeal carcinoma and other EBV+ solid malignancies. The company believes NAVAL-1 might support numerous NDA applications across diverse EBV+ lymphoma subtypes. The trial has sites open across the world, including the United States, Canada, Europe, and Asia. A report on the initial cohorts that may progress to Stage 2 of the study is expected in the fourth quarter of 2022.
Valemetostat, a potentially selective first-in-class dual inhibitor of EZH1 and EZH2, is now under clinical development in Daiichi Sankyo’s alpha portfolio. Valemetostat is an epigenetic dysregulation inhibitor that targets the EZH1 and EZH2 enzymes. The company announced the NDA filing in Japan for adult T-cell leukemia/lymphoma (ATL) patients in 2021. Valemetostat’s NDA filing in Japan is based on pivotal Phase II trial findings in Japanese patients with three aggressive subtypes of relapsed/refractory ATL.
Secura Bio’s COPIKTRA (Duvelisib) is a phosphoinositide 3-kinase inhibitor that is administered orally. The phosphoinositide 3-kinase (PI3K) signaling pathway is a major regulator of cancer proliferation (rapid expansion or spread) and metastasis (the development of new cancer growths distant from the initial site of cancer). Secura Bio stated that it had finished participation in the PRIMO trial in 2022. PRIMO has enrolled 157 participants in their study of COPIKTRA for relapsed or refractory (r/r) peripheral T-cell lymphoma treatment in adult patients.
Tolinapant is a synthetic, small molecule developed by Astex Pharma that acts as a dual antagonist of cellular inhibitor of apoptosis protein (cIAP) 1 and X-linked inhibitor of apoptosis protein (XIAP) and has been shown in nonclinical models to have potent proapoptotic and tumor growth inhibitory activity. Tolinapant is now being tested in patients with PTCL and CTCL as part of Phase II research in patients with advanced solid tumors and lymphomas to establish safety, pharmacokinetics, and preliminary efficacy. Astex Pharmaceuticals announced in August 2020 that the US FDA had approved Tolinapant (previously ASTX660) for the treatment of T-cell lymphoma.
AstraZeneca’s AZD4573 is a Cyclin-dependent kinase 9 (CDK9) inhibitor that is a transcriptional regulator and a possible therapeutic target for several malignancies. Multiple nonselective CDK9 inhibitors have advanced in clinical trials but have been hampered by a restricted therapeutic window. AZD4573, a new, potent, and highly specific CDK9 inhibitor, is described in this study. The company reported in Q1 2022 that the first patient began dosing in Q1 2022, and the company anticipated Phase II results by 2023.
Expected Roadblocks in the Peripheral T-cell Lymphoma Treatment Market
In comparison to the United States and Japan, Europe has a significant shortage of approved PTCL drugs. The current therapies are ineffective, and forthcoming therapies in the peripheral T-cell lymphoma treatment market encounter challenges in clinical trials due to the small number of patients with rare variants of PTCL.
Moreover, the high cost of new therapies may hinder their acceptance, particularly in cost-conscious economies undergoing austerity measures. Furthermore, diagnostic and treatment solutions are desperately needed in the peripheral T-cell lymphoma treatment market to enhance the prognosis of PTCL and patient outcomes in the future.
What Lies Ahead in the Peripheral T-cell Treatment Market?
Efforts have been made in recent years to incorporate novel treatments into combination methods for treating this difficult disease type. The recent introduction of therapies such as HIYASTA, DRAVIAS, and REMITORO in Japan has expanded the PTCL market and addressed unmet patient needs.
According to DelveInsight analysis, the total PTCL market size in the 7MM is USD 564 million in 2021 and is projected to grow during the forecast period (2022–2032).
The rapid change in the peripheral T-cell lymphoma treatment market is mainly attributed to the launch of Adcetris, along with the robust clinical pipeline and the upcoming launch of emerging therapies such as Azacitidine (CC-486) and COPIKTRA (Duvelisib) in the forecasted period (2022–2032).
Furthermore, several other PTCL therapies in development right now look to be significantly more promising and less hazardous than the peripheral T-cell lymphoma treatments that are already accessible to patients. As there is no authorized therapy for CHOP-ineligible individuals, firms can turn their emphasis to this patient group in the coming years.

FAQs
PTCL is a kind of aggressive lymphoma that develops from mature T-cells and natural killer (NK) cells. It accounts for 10-15% of all non-Hodgkin lymphomas globally. The WHO categorization system identifies PTCL subtypes and divides the disorders into three categories: nodal, extranodal, and leukemic.
The exact peripheral T-cell lymphoma causes are unknown; however, it has been linked to Epstein-Barr virus (EBV) or human T-cell leukemia virus-1 exposure (HTLV-1).
The PTCL symptoms in the patients are similar to those seen in other aggressive lymphoid tumors.
The current PTCL treatment consists of nonsurgical and surgical regimens, with the nonsurgical phase involving physical therapy and medicines. On the other hand, surgical protocols include various surgical operations such as discectomy or microdiscectomy, laminectomy, or laminotomy.
The overall peripheral T-cell lymphoma incidence and frequency vary geographically. However, PTCL, in general, is more common in Asia and the Caribbean.
Except for anaplastic lymphoma kinase-positive ALCL, most PTCL subtypes are aggressive lymphomas, including PTCL-NOS, AITL, ALCL, enteropathy-type T-cell lymphoma, and extranodal NK cell/T-cell lymphoma, with 5-year survival rates of less than 30%.
BELEODAQ (Belinostat, Acrotech Biopharma), ISTODAX (Romidepsin, Celgene), FOLOTYN (Pralatrexate, Acrotech Biopharma), ARRANON (Nelarabine, GlaxoSmithKline), and ADCETRIS (Brentuximab vedotin, Seattle Genetics) are among the FDA-approved peripheral T-cell lymphoma treatments.







-Agonist.png)


