Intuitive’s da Vinci 5 Gets CE Mark; Boston Scientific’s FARAPULSE™ Secures Expanded FDA Approval; Trividia Health’s TRUE METRIX® Added to Pennsylvania Medicaid List; Cerapedics’ PearlMatrix™ Sees First U.S. Clinical Use Post-FDA Nod; Smith+Nephew Expands Q-FIX◊ with Knotless Option; Cochlear Unveils World’s First Smart Cochlear Implant
Intuitive’s da Vinci 5 Surgical System Received CE Mark On July 02, 2025, Intuitive, widely…
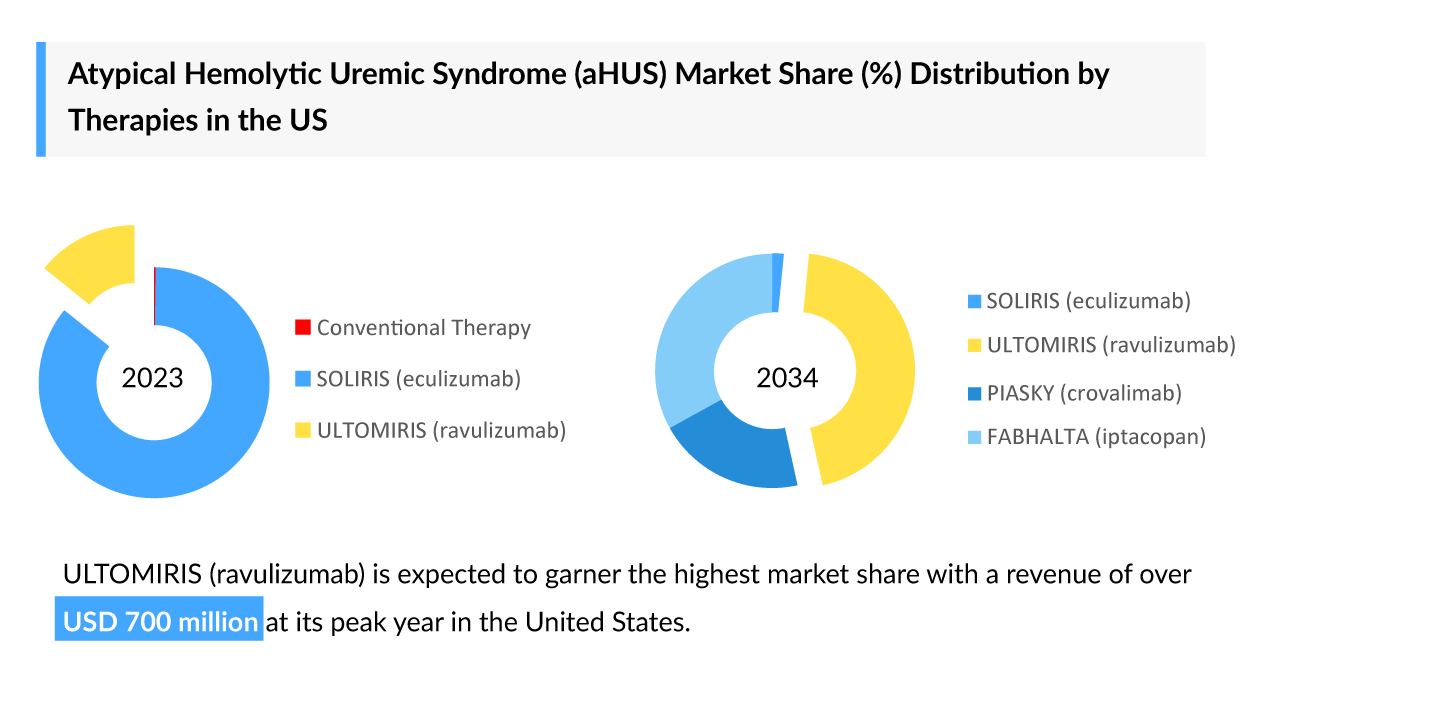
Polycythemia Vera: Growing Competition and the Changing Treatment Landscape in 2025
Articles
Jul 10, 2025
The Rise of Menstrual Cups: Revolutionizing Healthcare and Sustainability
Articles
Jul 09, 2025
SUNRISE Trial: Eptinezumab Delivers Robust Migraine Prevention in Predominantly Asian Population
Others
Jul 09, 2025








-Agonist.png)