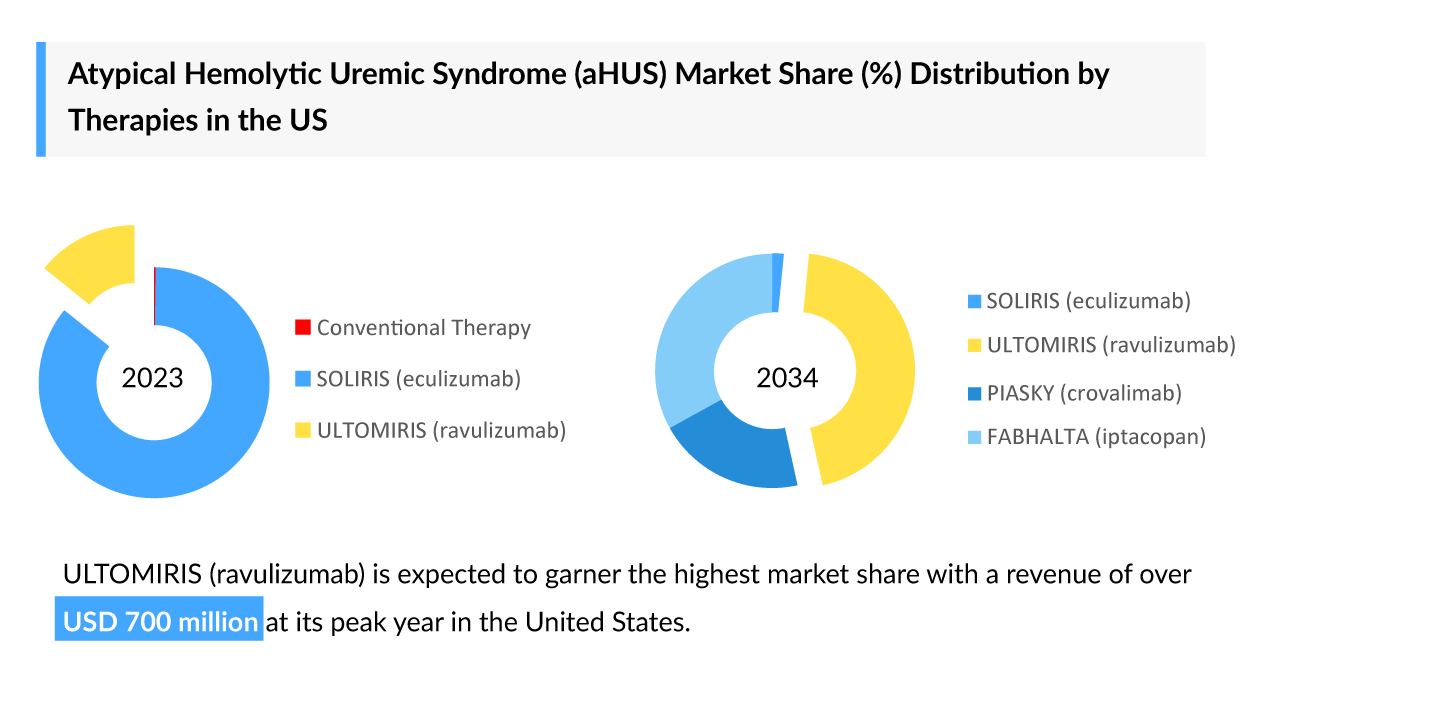
Merck’s WINREVAIR Granted FDA Priority Review for Pulmonary Arterial Hypertension; KalVista’s EKTERLY Approved by FDA as First Oral On-Demand Therapy for Hereditary Angioedema; Fujifilm’s FF-10832 Receives FDA Orphan Drug Designation for Biliary Tract Cancer; Mustang Bio’s MB-101 Granted FDA Orphan Drug Designation for Glioblastoma and Astrocytomas; Denali’s Tividenofusp Alfa Accepted for FDA Priority Review for Hunter Syndrome
Merck's WINREVAIR Gets FDA Priority Review for Pulmonary Arterial Hypertension Merck, also known as MSD…
Current Pheochromocytomas and Paragangliomas Treatment Paradigms: Challenges and Opportunities
Articles
Jul 07, 2025
Nanobots in Medicine: Transforming Healthcare from the Inside Out
Articles
Jul 06, 2025
What Impact Does Speech and Voice Recognition Technology Bring to the Healthcare System?
Others
Jul 04, 2025
Discover How Learning Disability Treatment Landscape is Evolving with the Emergence of Digital Assistant Technologies
Snippets - A Small piece of News or Article
Jul 04, 2025








-Agonist.png)